is a mathematical procedure that It involves combining the individual wave functions for atomic orbitals (pure) s and p to obtain wavefunctions for new atomic orbitals hybrids with different shapes and orientations.
HYBRIDIZATION sp3:
Blending a three 2s 2p
Hybridization called sp3
in each of the vertices marked "sp3" is the C atom to form bonds with H to form the methane molecule, with tetrahedral arrangement. Paired electrons occupy the region of the links which, in turn, form byte about C.
HYBRIDIZATION sp2:
It is defined as the combination of an orbital P S and 2 to form three hybrid orbitals, which are arranged on a plane angles of 120 °.
The atoms forming sp2 hybrids can form compounds with double bonds. Form an angle of 120 ° and the molecule is planar. A single bonds are referred to as sigma bonds (σ) and the double bonds are composed of a sigma bond and a pi bond (
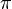 ).
).HYBRIDIZATION sp:
It is defined as the combination of an orbital Sy P, to form 2 hybrid orbital, linear orientation. This is the type of hybrid link with a 180 ° angle and that is existing in compounds with triple bonds and alkynes
It is characterized by the presence of two orbital pi (π).
Secondly we must know what is the isomerism
Structural isomerism:
It is a form of isomerism, where molecules with the same molecular formula have a different distribution of bonds between atoms, contrary to what happens in the stereoisomerism.
Space isomerism:
Exhibit stereoisomerism those compounds having identical molecular formulas atoms and have the same distribution, but their arrangement in space is different, that is, they differ in the spatial orientation of their atoms.
Likewise stereoisomers if they are plotted on a plane. It is necessary to represent them in space to visualize the differences. They can be of two types:
- Conformational isomerism:
isomerism in this type of conversion from one form to another is possible because the rotation around the axis of the bond formed by the carbon atoms is more or less free
- configurational isomerism
Not just a simple rotation to convert one form to another and although the spatial arrangement is the same, are not interconvertible isomers. It is divided into: Geometric or cis-trans isomerism and optical isomerism. Configurational isomers are isolable, since a large amount of energy is needed to interconvertirlos







No hay comentarios:
Publicar un comentario