Structural isomerism:
physical properties
- have different properties such as:
- Boiling point
- melting point
- density
- solubility:
for instance:
The n-butanol, boiling punt ° 1180
Isobutanol, boiling point 108 ° C
The atoms involved in a single bond can rotate freely without deterioration, but were not at a double bond, as if it happened, the p pi bond that forms the double bond is broken. Therefore, some alkenes may exist in two different forms, provided that each carbon double bond having different substituent groups
space isomerism
studied organic compounds with the same structural formula but different arrangements of atoms in space.
These isomers differ only in the physical properties.
- geometrical isomerism presented with double bond compounds: carbon - carbon
- the eteroisomeros only be faithfully represented by models in space or perspective drawing
- optical isomerism: Physical and chemical properties have igualesm differ only in the property to divert polarized light
- the enantiomers their mirror images are not superimposable one another, ie are chiral molecules
- conformational isomers are structurally stable, meaning their atoms can not be swapped freely around the links that require the breaking and sharing of atom


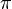 ).
).

















